October 29, 2025

In our last article, we discussed how “Healthy Aging” is emerging as a new, targeted therapeutic area. Among the most surprising candidates showing promise in this field are glucagon-like peptide-1 receptor agonists (GLP-1 RAs) – medications originally developed to target diabetes. Research into GLP-1 RAs has an interesting history that started back in the early 1900s and involved the study of Gila monster venom. Joe Schwartz at the Montreal Gazette has a great article detailing the path to the discovery of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) and how they influenced insulin release after meals. Unfortunately, these peptide hormones persist in the bloodstream for only minutes in their native form, making them impractical as therapeutic agents. But further research led to the development of longer-acting peptides that mimic their mechanism of action. Labeled as a new class of drugs, called “incretin mimetics”, Eli Lilly’s Exenatide (Byetta®) was the first GLP-1RA to be approved by the FDA, as an add-on therapy for patients taking other forms of glucose-control medications, in 2005.
Over the last two decades, longer-acting formulations have been developed that eliminated the need for twice-daily injections. These advances improved dosing schedules from daily to weekly, with oral formulations now available as well. The research revealed an unexpected bonus: dramatic weight loss that is even more pronounced in people without Type 2 Diabetes [1]. Noting the side-benefit of weight loss from this new class of drugs, Liraglutide became the first FDA-approved GLP-1RA for obesity in 2014, and Semaglutide (Wegovy®) followed with approval in 2021. Beyond weight loss and glucose control, clinical trials are now showing benefits that extend beyond these two targets.

GLP-1 RAs work by mimicking a gut hormone that regulates blood sugar and appetite [2]. But robust clinical trial data now shows that these drugs also significantly reduce the risk of major cardiovascular events like heart attack and stroke, reducing mortality in patients with type 2 diabetes [3-6]. This benefit extends beyond what would be expected from weight loss or glucose control alone, suggesting a direct protective effect on the cardiovascular system.

In addition to their cardiovascular impacts, GLP-1 RAs have been shown to reduce liver fat buildup in non-alcoholic fatty liver disease (NAFLD) [7], and slow the progression of chronic kidney disease (CKD) in those with type 2 diabetes [8]. The FDA has already expanded approvals for semaglutide to include cardiovascular risk reduction and CKD protection, which are classic age-related conditions. This alone positions them as powerful “healthspan-extending” agents, and with newer, potentially more powerful classes of glucagon-modulating medications on the horizon, the future seems promising.
The triple receptor (GLP-1/GIP/GCG*) agonist retatrutide (not yet FDA-approved) not only demonstrated greater weight-loss efficacy over semaglutide in obese individuals without diabetes, but also improved outcomes in diabetic patients with steatotic liver disease (fatty liver). After 24 weeks of 12 mg retatrutide treatment, 86% of patients with type 2 diabetes and liver steatosis saw their liver fat return to healthy levels [9].
It has been generally accepted in the geroscience community that anti-aging drugs must target the fundamental “hallmarks of aging” [10], so where do GLP-1RAs fit in?
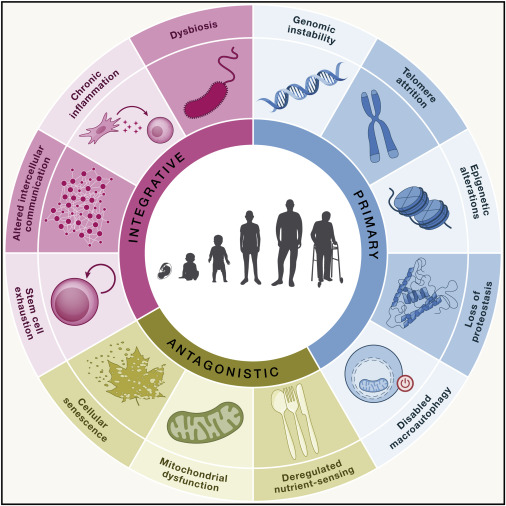
Hallmarks of Aging, (López-Otín et al., 2023. “Hallmarks of aging: An expanding universe“, Cell)
At present, these drugs target at least 4 hallmarks:

While excitement about the various potential disease targets for GLP-1 agonists is encouraging, it’s important to understand that these drugs are not without side-effects. Beyond the well-known side effects like gastrointestinal issues, pancreatitis, and hypotension, there are specific risks to consider from a longevity perspective, including:

GLP-1 RAs are arguably the most compelling candidates for a “longevity-enhancing” drug to date, as they directly impact multiple core drivers of aging. Their ability to protect the heart, kidneys, and liver is undeniable. However, they are not a simple “anti-aging” pill, and the significant risk of muscle and bone loss presents a major hurdle that must be addressed. Further research is also needed to understand the neurological impacts of GLP-1 RAs. A key priority is to identify classes of GLP-1 RAs that can more effectively cross the blood-brain barrier.
GLP-1 RAs are not a cure for aging, but they ARE a groundbreaking class of drugs that are leading a paradigm shift in medicine. We are living longer now than we did at the start of this century, but we are also spending more time in poor health in our final years [30]. This class of drugs is rapidly moving from treating single diseases to targeting the aging process itself, with the promise of extending not just lifespan but more importantly, healthspan in our final decades.
Disclaimer: The mention of specific companies, products, or organizations in this article is for informational purposes only and does not imply endorsement. The companies referenced were not consulted, involved in the preparation of this content, nor did they provide any funding or compensation.
References
[2] A. Moiz, K. B. Filion, M. A. Tsoukas, O. H. Y. Yu, T. M. Peters, and M. J. Eisenberg, “Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation,” Am. J. Med., vol. 138, no. 6, pp. 934–940, 2025, doi: https://doi.org/10.1016/j.amjmed.2025.01.021.
[3] C. Brinkmann, “Interaction Between Non-Insulin Glucose-Lowering Medication and Exercise in Type 2 Diabetes Mellitus – New Findings on SGLT2 Inhibitors.,” Front. Endocrinol. (Lausanne)., vol. 12, p. 694099, 2021, doi: 10.3389/fendo.2021.694099.
[4] A. Seminer et al., “Cardioprotective Glucose-Lowering Agents and Dementia Risk: A Systematic Review and Meta-Analysis,” JAMA Neurol., vol. 82, no. 5, pp. 450–460, May 2025, doi: 10.1001/jamaneurol.2025.0360.
[5] F. B. Rivera et al., “Cardiovascular and renal outcomes of glucagon-like peptide 1 receptor agonists among patients with and without type 2 diabetes mellitus: A meta-analysis of randomized placebo-controlled trials.,” Am. J. Prev. Cardiol., vol. 18, p. 100679, Jun. 2024, doi: 10.1016/j.ajpc.2024.100679.
[6] “Unlocking the broad health benefits and risks of GLP-1 receptor agonist drugs,” Nat. Med., vol. 31, no. 3, pp. 745–746, 2025, doi: 10.1038/s41591-024-03476-8.
[7] R. Nevola et al., “GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives.,” Int. J. Mol. Sci., vol. 24, no. 2, Jan. 2023, doi: 10.3390/ijms24021703.
[8] E. R. Webster, A. Perkovic, B. L. Neuen, K. R. Tuttle, and V. Perkovic, “Effects of anti-inflammatory agents on clinical outcomes in people with chronic kidney disease: a systematic review and meta-analysis of randomized control trials,” Clin. Kidney J., vol. 18, no. 3, p. sfaf001, Mar. 2025, doi: 10.1093/ckj/sfaf001.
[9] A. J. Sanyal et al., “Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial,” Nat. Med., vol. 30, no. 7, pp. 2037–2048, 2024, doi: 10.1038/s41591-024-03018-2.
[10] C. López-Otín, M. A. Blasco, L. Partridge, M. Serrano, and G. Kroemer, “Hallmarks of aging: An expanding universe.,” Cell, vol. 186, no. 2, pp. 243–278, Jan. 2023, doi: 10.1016/j.cell.2022.11.001.
[11] S. H. Alharbi, “Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications.,” Ther. Adv. Endocrinol. Metab., vol. 15, p. 20420188231222370, 2024, doi: 10.1177/20420188231222367.
[12] C. K. Wong et al., “Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation,” Cell Metab., vol. 36, no. 1, pp. 130-143.e5, Jan. 2024, doi: 10.1016/j.cmet.2023.11.009.
[13] J. A. Amorim, G. Coppotelli, A. P. Rolo, C. M. Palmeira, J. M. Ross, and D. A. Sinclair, “Mitochondrial and metabolic dysfunction in ageing and age-related diseases,” Nat. Rev. Endocrinol., vol. 18, no. 4, pp. 243–258, 2022, doi: 10.1038/s41574-021-00626-7
[14] C. Luna-Marco et al., “Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes.,” Redox Biol., vol. 66, p. 102849, Oct. 2023, doi: 10.1016/j.redox.2023.102849.
[15] M. Y. Kang, T. J. Oh, and Y. M. Cho, “Glucagon-Like Peptide-1 Increases Mitochondrial Biogenesis and Function in INS-1 Rat Insulinoma Cells.,” Endocrinol. Metab. (Seoul, Korea), vol. 30, no. 2, pp. 216–220, Jun. 2015, doi: 10.3803/EnM.2015.30.2.216.
[16] V. J. Old, M. J. Davies, D. Papamargaritis, P. Choudhary, and E. L. Watson, “The Effects of Glucagon-Like Peptide-1 Receptor Agonists on Mitochondrial Function Within Skeletal Muscle: A Systematic Review,” J. Cachexia. Sarcopenia Muscle, vol. 16, no. 1, p. e13677, Feb. 2025, doi: https://doi.org/10.1002/jcsm.13677.
[17] K. O. Kopp, E. J. Glotfelty, Y. Li, and N. H. Greig, “Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment.,” Pharmacol. Res., vol. 186, p. 106550, Dec. 2022, doi: 10.1016/j.phrs.2022.106550.
[18] I. Salcedo, D. Tweedie, Y. Li, and N. H. Greig, “Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders.,” Br. J. Pharmacol., vol. 166, no. 5, pp. 1586–1599, Jul. 2012, doi: 10.1111/j.1476-5381.2012.01971.x.
[19] J. L. Cummings et al., “evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating efficacy, safety, and tolerability of semaglutide in early-stage symptomatic Alzheimer’s disease,” Alzheimers. Res. Ther., vol. 17, no. 1, p. 14, 2025, doi: 10.1186/s13195-024-01666-7.
[20] W. Peng, R. Zhou, Z.-F. Sun, J.-W. Long, and Y.-Q. Gong, “Novel Insights into the Roles and Mechanisms of GLP-1 Receptor Agonists against Aging-Related Diseases,” Aging Dis., vol. 13, no. 2, pp. 468–490, Apr. 2022, doi: 10.14336/AD.2021.0928.
[21] I. J. Neeland, J. Linge, and A. L. Birkenfeld, “Changes in lean body mass with glucagon-like peptide-1-based therapies and mitigation strategies.,” Diabetes. Obes. Metab., vol. 26 Suppl 4, pp. 16–27, Sep. 2024, doi: 10.1111/dom.15728.
[22] J. W. Mastaitis et al., “GDF8 and activin A blockade protects against GLP-1–induced muscle loss while enhancing fat loss in obese male mice and non-human primates,” Nat. Commun., vol. 16, no. 1, p. 4377, 2025, doi: 10.1038/s41467-025-59485-9.
[23] E. Çetin, B. Pedersen, and M. F. Burak, “Paradigm shift in obesity treatment: an extensive review of current pipeline agents.,” Turkish J. Med. Sci., vol. 55, no. 1, pp. 1–16, 2025, doi: 10.55730/1300-0144.5938.
[24] J. P. Brito et al., “GLP-1RA Use and Thyroid Cancer Risk.,” JAMA Otolaryngol. Head Neck Surg., vol. 151, no. 3, pp. 243–252, Mar. 2025, doi: 10.1001/jamaoto.2024.4852.
[25] S. M. Baxter et al., “Glucagon-Like Peptide 1 Receptor Agonists and Risk of Thyroid Cancer: An International Multisite Cohort Study,” Thyroid®, vol. 35, no. 1, pp. 69–78, Jan. 2025, doi: 10.1089/thy.2024.0387.
[26] F. Salvo and J.-L. Faillie, “GLP-1 Receptor Agonists and Suicidality—Caution Is Needed,” JAMA Netw. Open, vol. 7, no. 8, pp. e2423335–e2423335, Aug. 2024, doi: 10.1001/jamanetworkopen.2024.23335.
[27] G. Schoretsanitis, S. Weiler, C. Barbui, E. Raschi, and C. Gastaldon, “Disproportionality Analysis From World Health Organization Data on Semaglutide, Liraglutide, and Suicidality,” JAMA Netw. Open, vol. 7, no. 8, pp. e2423385–e2423385, Aug. 2024, doi: 10.1001/jamanetworkopen.2024.23385.
[28] J. Huang et al., “Functional and multi-omic aging rejuvenation with GLP-1R agonism,” bioRxiv, p. 2024.05.06.592653, Jan. 2024, doi: 10.1101/2024.05.06.592653.
[29] B. V Ineichen, E. Furrer, S. L. Grüninger, W. E. Zürrer, and M. R. Macleod, “Analysis of animal-to-human translation shows that only 5% of animal-tested therapeutic interventions obtain regulatory approval for human applications.,” PLoS Biol., vol. 22, no. 6, p. e3002667, Jun. 2024, doi: 10.1371/journal.pbio.3002667.
[30] A. Garmany and A. Terzic, “Healthspan-lifespan gap differs in magnitude and disease contribution across world regions,” Commun. Med., vol. 5, no. 1, p. 381, 2025, doi: 10.1038/s43856-025-01111-2.